One partner. End-to-end success.
Complexity, risk, and rigor. No regulated medical device gets to market without facing the gauntlet.
As a leading medical device CDMO, we offer medical device contract manufacturing, medical device prototyping, and medical device assembly services — all under one roof.
The most important first step is to protect your medical device journey with the most capable partner. Global med-tech innovators turn to Biorep.
Why Biorep?
Contract design and manufacturing of medical devices is all we’ve been doing for over 30 years.
- End-to-end capabilities
From concept through scale-up and launch, we cover every stage of regulated device development. - Deep domain experience
Focused exclusively on medical devices means we bring mature processes and risk mitigation to every engagement. - Regulatory & quality infrastructure
ISO 13485 certified and FDA-registered, we ensure the systems and controls necessary to satisfy global regulators. - Collaborative, flexible partnerships
Fully formed design? Or just an idea? Your team plus ours means tailored engagement models.
Core Services
We structure our offerings so you can pick precisely what you need — or let us do it all.
Medical Device Prototyping & Early Development
Validate form, fit, functionality, manufacturability, and user interface features, fast. Biorep’s rapid prototyping capabilities include rapid iteration, design for manufacturability (DFM) review, tolerancing, and early risk reduction.
We work closely in design control phases so that prototypes progressively mature into manufacturable designs. This helps you de-risk later stages.

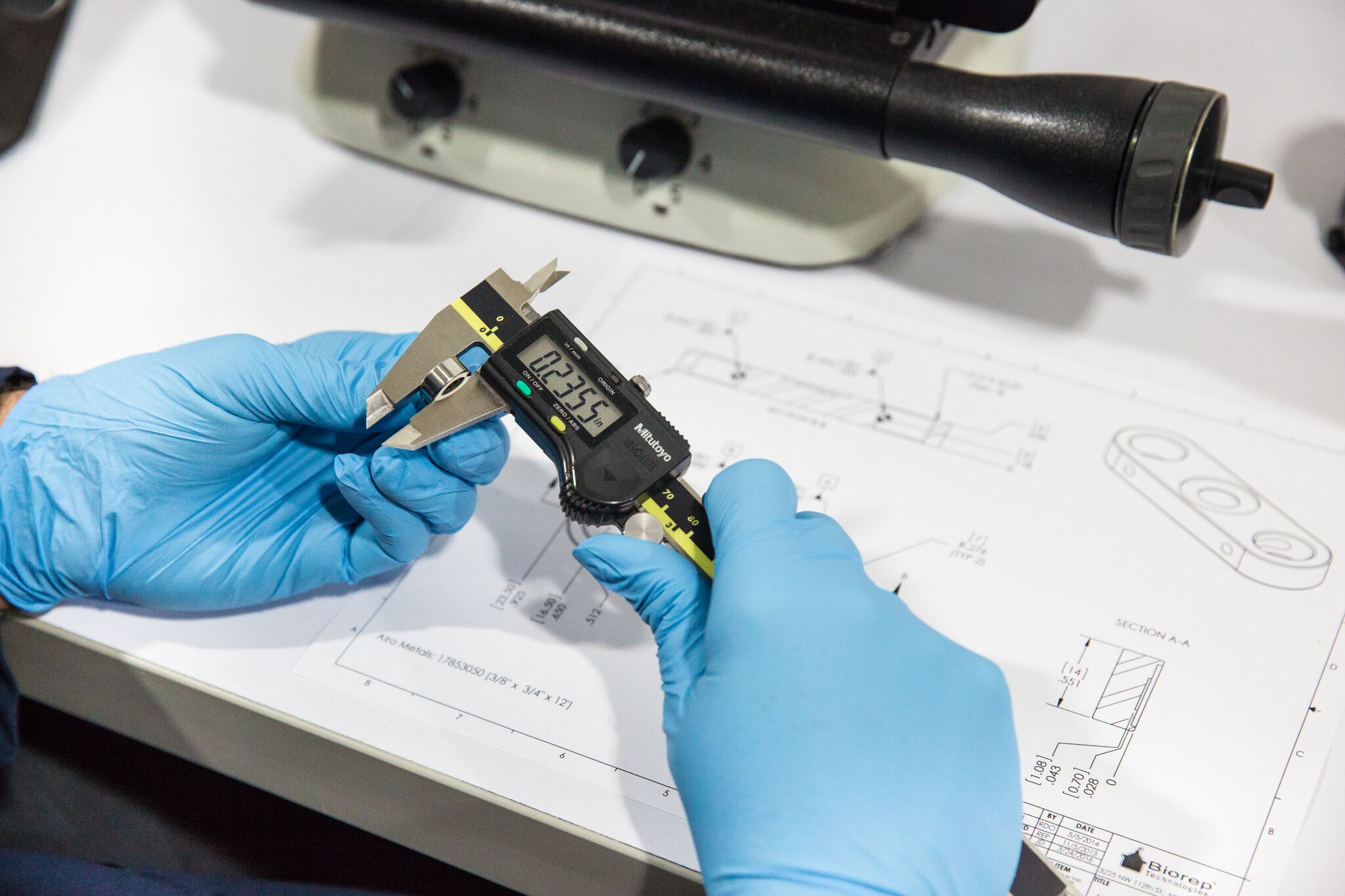
Medical Device Contract Manufacturing (Full-Scale Production)
Once your design is ready for scale, our medical device contract manufacturing capability is your bridge to robust, high-yield production. We focus on:
- Process validation
- Traceability & serialization
- Supply chain sourcing and vendor quality
- Equipment qualification, calibration, maintenance
- Change control and CAPA management
Our vertically integrated structure minimizes handoffs and simplifies oversight. We become an extension of your engineering and quality teams.
Medical Device Assembly Services
Complex subassemblies, final device integration, and test staging all demand high precision and cleanroom discipline. Our medical device assembly services include:
- Multi-component assembly (mechanical, micro-mechanical, electromechanical)
- Automated or manual assembly depending on volume and complexity
- In-process inspection, functional and environmental testing
- Packaging interface and labeling readiness
Having assembly in-house means greater control, faster cycle times, and better responsiveness to change.

CDMO Partnerships & Hybrid Models
As a full medical device CDMO, Biorep can act as your single-source development and manufacturing partner — or you can adopt hybrid models (you lead design, we execute manufacturing, or vice versa). This flexibility is critical when working with global med-tech firms who may want to retain some capabilities or manage IP internally.
Typical Client Scenarios & Use Cases
Scenario
New device concept or spinout
Mid-stage product lacking scale
Complex product needing final assembly
Strategic outsourcing to CDMO
Biorep Role
Lead prototyping, risk analysis, regulatory planning
Take on contract manufacturing
Integrate full assembly services
Full turnkey development + lifetime manufacturing
Benefits
Reduce time to first viable prototype
Scale volumes, improve yield, reduce cost
Improve control and responsiveness
Consolidate vendor risk, simplify supplier management
For instance, we’ve supported cardiovascular and minimally invasive instrument programs, ENT devices, surgical tools, and more.
Working with Biorep: Our Process & Value Drivers
Discovery & Risk Assessment
Our first step is mapping your technical challenges, regulatory landscape, and roadmap. Early alignment reduces surprises later.
Prototyping & Design Verification
Rapid prototyping with feedback loops helps validate subsystems, materials, and functionality before committing to tooling.
Process Development & Validation
We industrialize your design—developing processes, control plans, equipment, layout, and documentation.
Scale Manufacturing & Assembly
As demand ramps, we scale from pilot to production volumes, executing medical device contract manufacturing and assembly services under tightly controlled systems.
Sustaining, Change Control & Lifecycle Support
After launch, we manage component obsolescence, enhancements, regulatory updates, and managed change control.
Across each stage, our goal is to deliver device performance, cost efficiency, quality compliance, and schedule integrity.
Why Global Med-Tech Leaders Choose Us
- Domain focus: We do only medical devices — no distractions, no mismatched priorities.
- Reduced vendor count: Handling prototyping, manufacturing, and assembly in one partner means fewer transitions, lower overhead, and better alignment.
- Risk mitigation: With documented design controls, validation history, and traceability baked in, you minimize surprise findings or regulatory rework.
- Scalable capacity: Whether you need one-off prototypes or tens of thousands of units globally, we scale accordingly.
- Global mindset: Our processes and regulatory systems are compatible with major markets (US, EU, Asia), positioning you for international rollout.

Next Steps & How We Engage
Reach out
Let’s schedule a technical discovery workshop. Share your device concept, challenges, or draft designs.
Scope proposal
We’ll propose phased deliverables—prototype, pilot, production—with clear milestones, risks, and budget.
Kickoff execution
Once engaged, we embed cross-functional teams to manage design, manufacturing, quality, and supply chain.
If you’re a global med-tech company evaluating outsourcing, or seeking to consolidate supply chain complexity, contact Biorep to explore how our medical device contract manufacturing, medical device prototyping, medical device assembly services, and comprehensive medical device CDMO structure can accelerate your success.
Biorep: your partner for innovation, execution, and growth.




