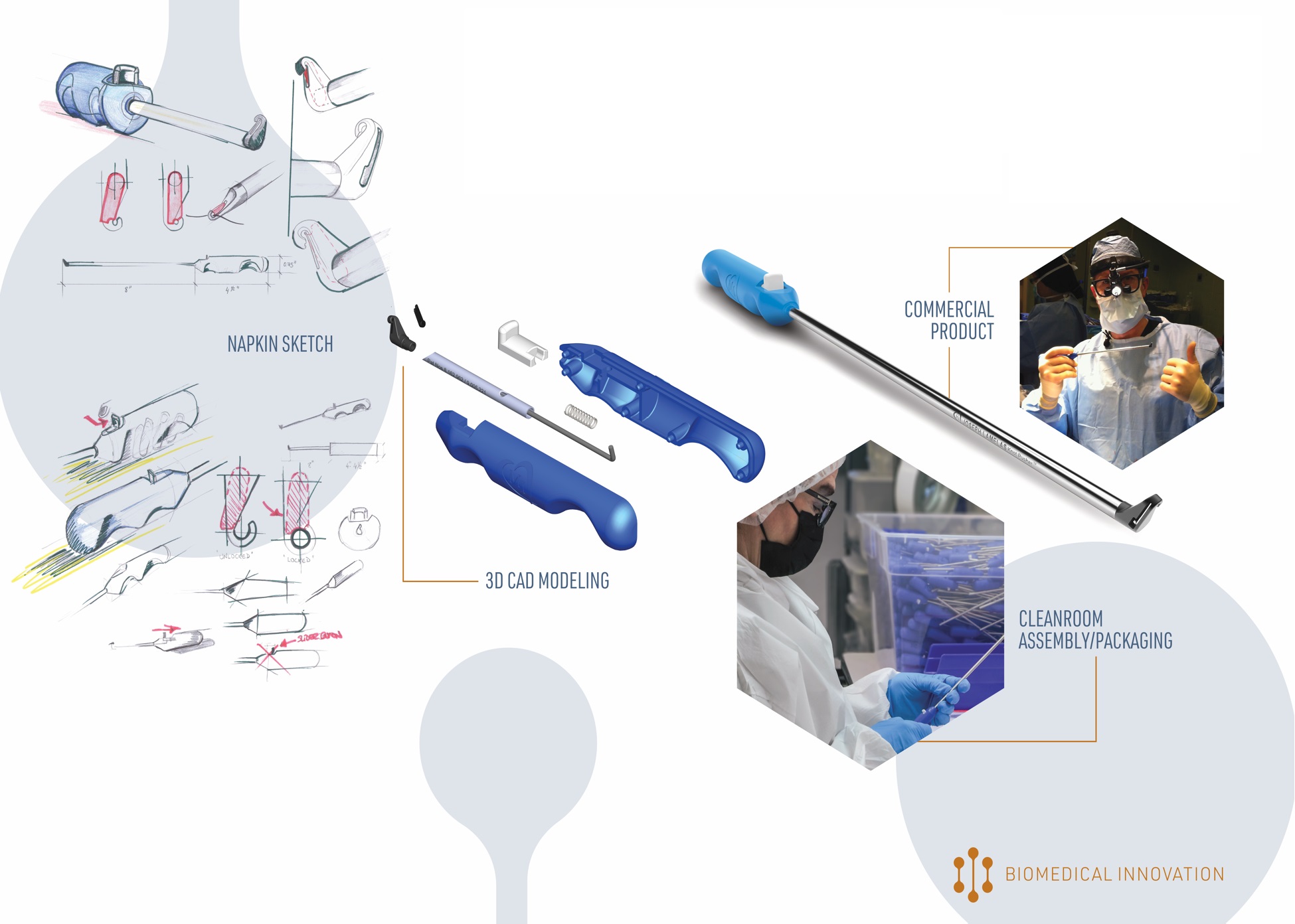
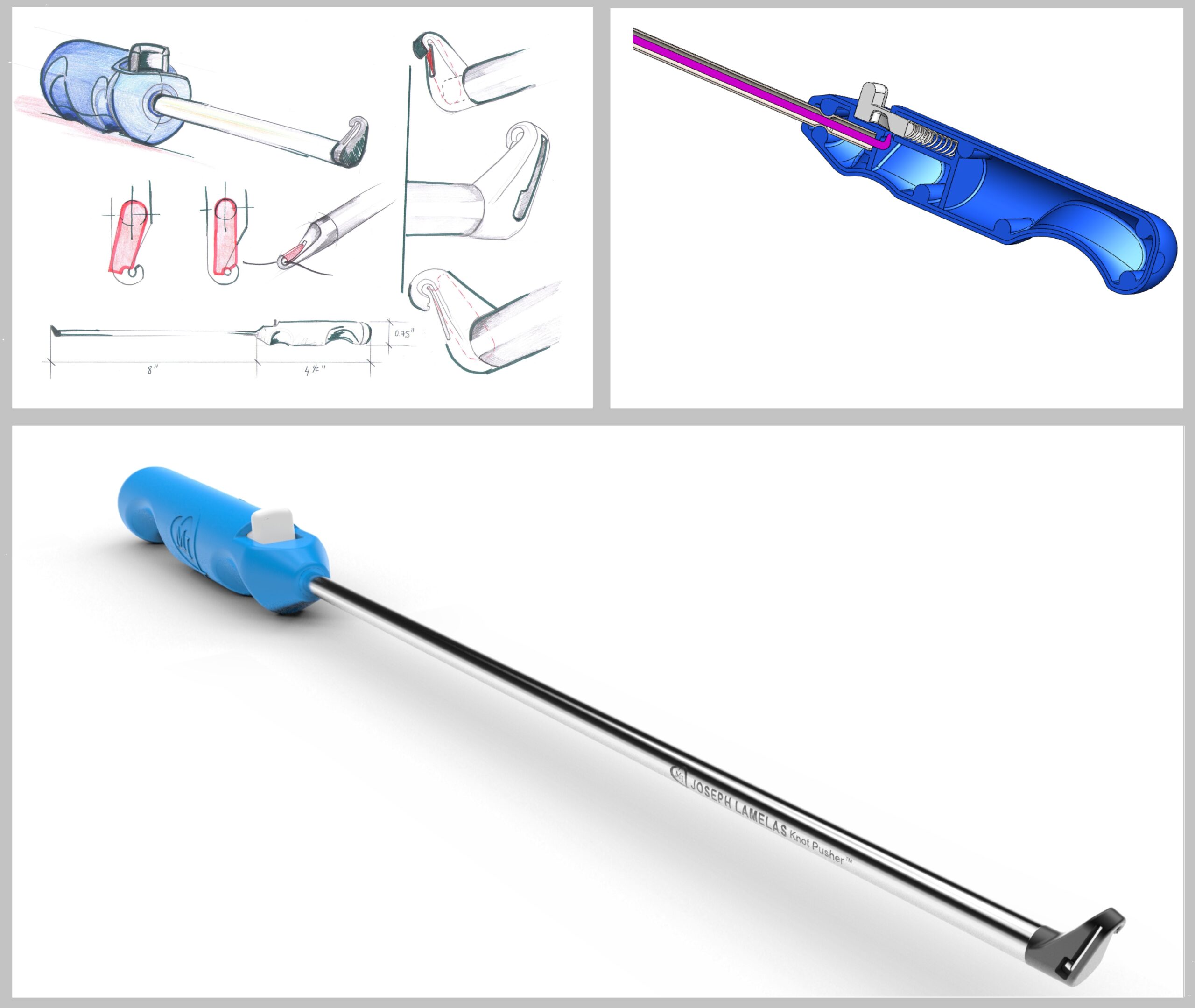
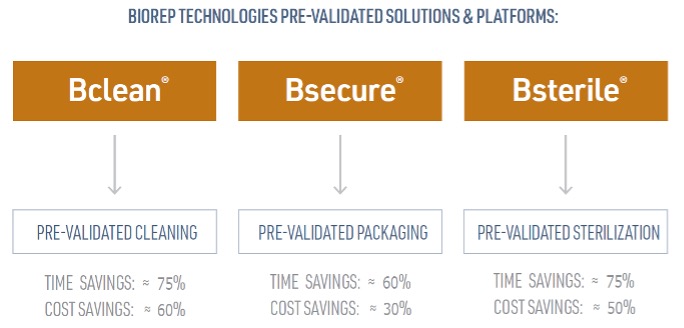
Two of the biggest hurdles to getting a new medical device into the market are the time and expense that it takes to validate a cleaning protocol, design and validate a medical device packaging system, and validate a sterilization process.
Our solutions save a valuable amount of time, money, and resources. This provides our customers a more efficient path to bring their products to market.
“Our pre-validated solutions save a valuable amount of time, money, and resources. This provides our customers a more efficient path to bring their products to market.”
– Felipe Echeverri, CEO, Biorep Technologies Inc.
Bclean® is a family of validated cleaning protocols that have been shown to be effective in removing manufacturing residues from CNC machined and injection molded components. The protocols cover complex part geometries with the most common engineering resin families. Most medical device components will be typically covered under one of our Bclean® pre-validated protocols.
Our BCLEAN® capabilities include:
- Ultrapure DI water system
- Ultrasonic cleaning
- IPA wipe or dip
- Cleaning process validation studies
- Cleaning validations for product families
Bsterile® is a family of gamma and Ethylene Oxide (EtO) sterilization validations for different bioburden counts at different sterilization dosages and ranges. Testing includes a determination of bioburden, bioburden recovery method validation, and bacteriostasis/fungistasis testing.
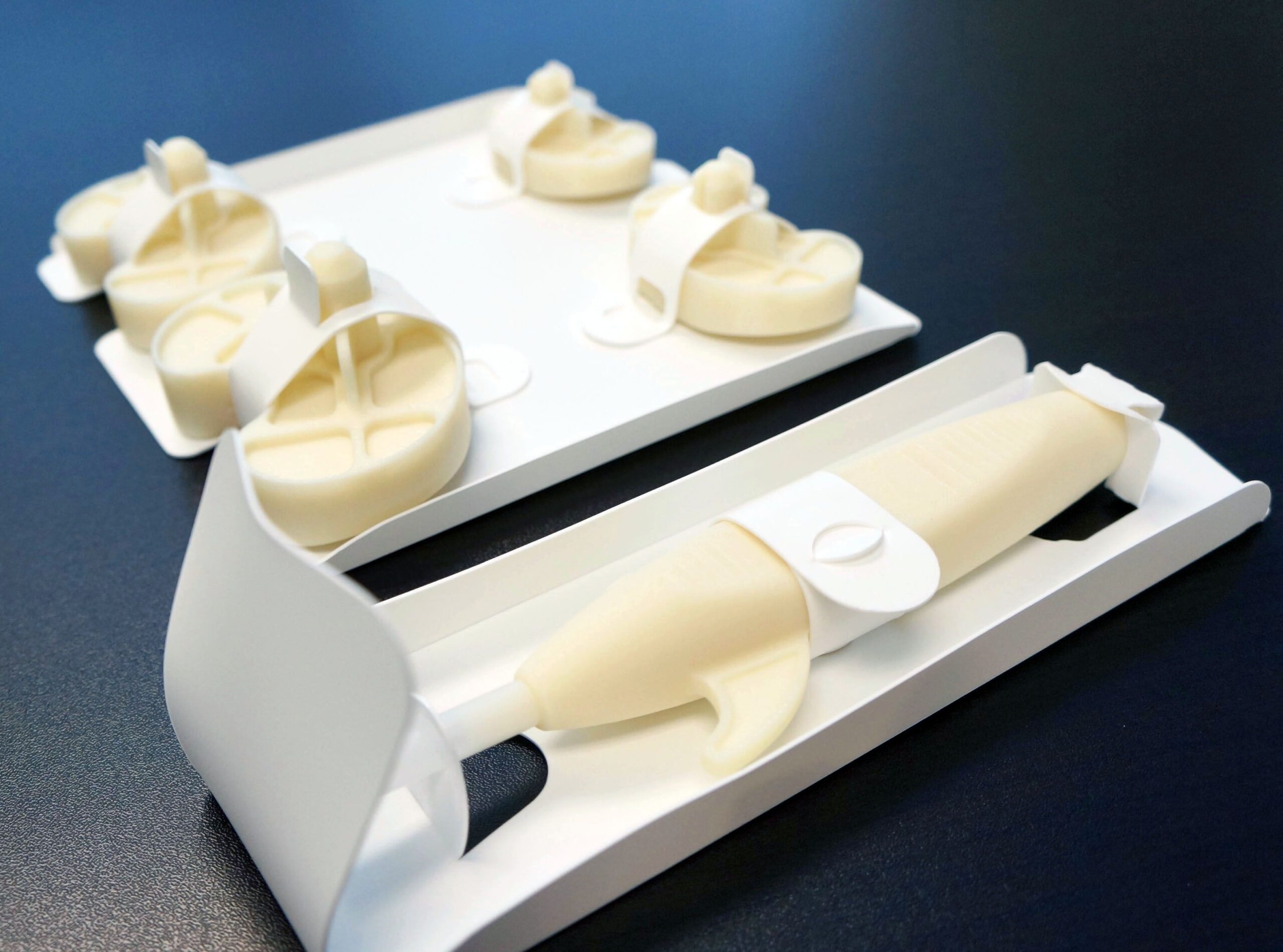
Bsecure® pouches can be customized to the needs of a specific component by adjusting the length and width of the pouch. This flexibility of customization offers a wide range of options for pre-validated packaging.
A seal validation has been completed with Poly-Tyvek® pouches for the Bsecure® offering, along with 3-year accelerated aging testing with Gamma sterilization and complete validation reports.
Bsecure® offers flexible package options, including Tyvek® lid stock, standard label stock, IFU paper stock and SBS shelf boxes to deliver a complete package system.